IEC 62304 defines the life cycle requirements for medical device software. The set of processes, activities, and tasks described in this standard establishes a common framework for medical device software life cycle processes that is similar to other safety-critical software development standards.
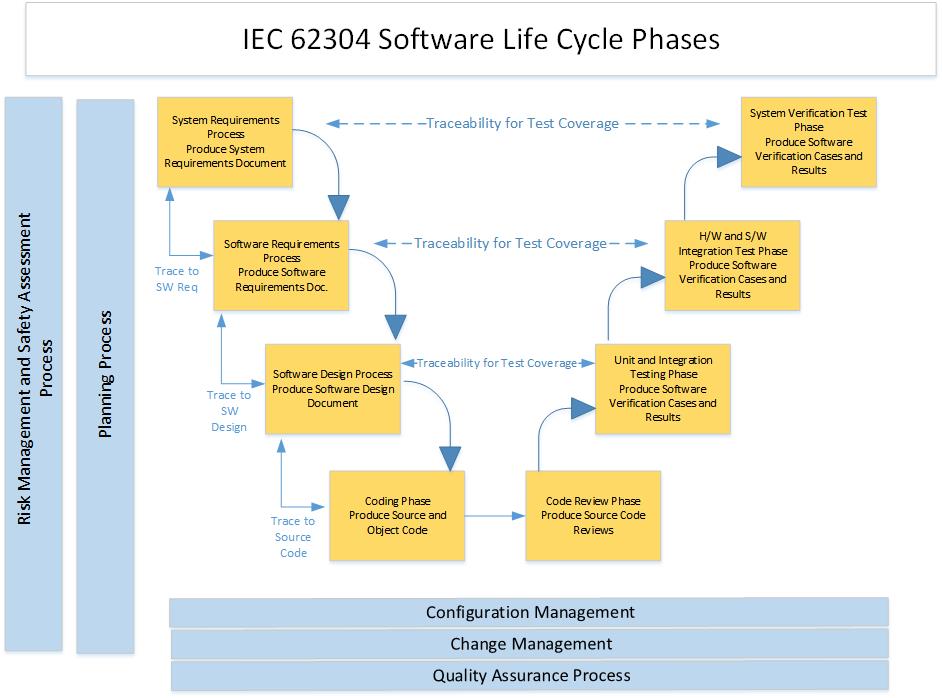
The software development life cycle model spans the life of the software from definition of requirements to release for manufacturing, which:
-
identifies the process, activities and tasks involved in development of a software product,
-
describes the sequence of and dependency between activities and tasks, and
-
identifies the milestones at which the completeness of specified deliverables is verified.
The following diagram provides a representation of the software life cycle phases, outputs, linkages and activities. The life cycle is framed by a planning process where a consistent set of plans and standards is used to describe the activities and phases. The life cycle is supported by a configuration management process, a change management process, and software quality assurance.